
Childhood Diabetic Issues 3.0 CE Hours
Course Objectives
- Develop an understanding regarding the prevalence of pediatric diabetes
- Understand the physiology involved with the development of diabetes
- Understand the difference between Type 1 and Type 2 diabetes
- Recognize the clinical presentation of hypoglycemic and hyperglycemic patients
- Develop a thorough understanding regarding the treatment of pediatric diabetes in the field.
Introduction
Diabetes continues to be a staggering healthcare problem. The prevalence of diabetes presents challenges for patients, healthcare providers, and the overall healthcare system. It is estimated that nearly 26 million people, or 8.3 percent of the U.S. population, has diabetes. Of those 26 million patients, 7 million are undiagnosed. The financial costs of this disease are approaching $200 billion annually, which represents one-third of all Medicare expenditures.
Approximately 2 million Americans are diagnosed with diabetes annually and, unlike most other major diseases, the incidence of this disease is increasing significantly. The number of new diagnoses of diabetes has tripled in the past three decades. Much of this growth is related to an increased incidence of Type 2 diabetes, a syndrome that is largely associated with poor lifestyle choices.
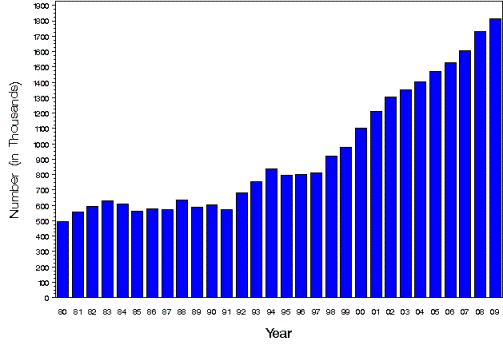
New Cases of Diagnosed Diabetes – Centers for Disease Control and Prevention
Of the 26 million diabetics, 215,000 patients are under 20 years of age. Approximately one in every 400 children or adolescents has the disease. Worldwide trends in pediatric diabetes younger age of disease development and increases in both Type 1 and Type 2 diabetes. Ancillary disease processes associated with diabetes include heart disease, hypertension, renal disease, neuropathies, non-traumatic amputations, and ocular disease. The presence of heart disease and hypertension among diabetics is two to four times higher than the general population.
Diabetes is the seventh leading cause of death in the U.S. and was a major contributing factor in 231,404 deaths in the latest year of available data. Mortality associated with Type 1 diabetes remains significant. A University of Pittsburgh cumulative study found that over fifteen percent of Type 1 diabetics, with childhood onset, will die by age 40 (a rate that is twenty times higher than the remainder of the population). One anomaly with pediatric diabetes is that patients who have onset of the disease prior to puberty have lower mortality rates than those who have onset during or closer to puberty (peripubertal).
The causes of death for Type 1 diabetics vary based on the amount of time the patient has had the disease. In the initial decade after diagnosis, coma is the leading cause of death. In the next decade, renal failure becomes the leading cause of death. For Type 1 diabetics who have survived the disease for two decades or more, cardiovascular disease becomes the leading cause of death.
Physiology
There are many variations of the definition, but metabolism can broadly be defined as the set of chemical processes needed to maintain life in a human being. A primary component of metabolism is enzymes. Enzymes are molecules that act as a catalyst to control the various metabolic processes. While many organs play a role in the metabolic process, the primary organs involved in diabetes mellitus are the pancreas, kidneys, and liver. A majority of the body’s energy comes from carbohydrate consumption and breakdown. One of the most basic needs for life is the ability to convert caloric intake into energy for cells.
Glycolysis is a very important process which involves converting caloric intake into cellular energy. This ten-step, complex chemical process involves the breakdown of sugars and releases major energy compounds such as adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NADH). The rate of glycolysis is regulated primarily in the liver, based on the current needs for more ATP and the appropriate blood sugar levels required. One of the byproducts of this regulatory mechanism is the conversion of excess energy to glycogen for hepatic storage. Glycogen plays a crucial role in the energy reserves in the human body during periods of fasting or low blood sugar levels. It is important to note that glycogen stores are lower in pediatrics and, therefore, a child’s ability to tap into the body’s energy reserves is lower.
The pancreas is a multifaceted organ which is crucial for the body’s ability to process food and convert it to energy. The pancreas is comprised of approximately one million small clusters of cells known as the Islets of Langerhans.
The islets themergency medical personnelelves produce four primary secretions which include the following:
- Alpha cells – secrete glucagon to increase blood glucose levels
- Beta cells – produce insulin to lower blood glucose levels
- Somatostatin – released by the pancreas to regulate alpha and beta cells
- Pancreatic polypeptide – the least-known chemical secreted by the pancreas. This secretion is released to regulate alpha and beta cells in order to manage blood glucose levels
Via different mechanisms, the pancreas also assists in the production of digestive enzymes to assist in the breakdown of food in the stomach and small intestine. The amount of each chemical released by the pancreas is directly tied to food intake and the homeostatic needs of the body based on hepatic input, blood sugar levels, and energy requirements.
Pancreatic Disease
Although diabetes is by far the most well known disease process that directly affects the pancreas among the pediatric population, pancreatitis, cancer (primary or secondary), and cystic fibrosis are also culprits in pancreatic dysfunction of non-traumatic origin. Additionally, congenital defects are a possible culprit in early pancreatic dysfunction or pancreatitis.
The pancreas is crucial for life maintenance, and its importance cannot be overlooked by the clinician.
Pancreatitis is a broad term indicating an inflammation of the pancreas. Pancreatitis in the pediatric population is rare, but should not be overlooked by the clinician because it carries a significant mortality rate, depending on etiology. The primary culprits of pediatric pancreatitis are trauma, virus, related ductal disease (often originating from the hepatic or biliary tract system), and pharmacological toxicity.
Nearly all patients with pancreatitis present with complaints of moderate to severe abdominal pain (and tenderness). Associated abdominal distention and vomiting are also common presentations. Symptoms are often made worse by eating. Pancreatitis can cause hypoglycemia or hyperglycemia and will often be on the list of differential diagnoses for most pediatrics presenting with new onset of diabetes symptomatology.
Hemorrhagic pancreatitis is a rare and potentially lethal form of pancreatitis with a mortality rate near fifty percent. This condition is an absolute critical life-threatening emergency. Due to the possibility of insult to major vasculature, surgical consult/intervention is required for the hemorrhagic pancreatitis patient.
Two ominous signs associated with hemorrhagic pancreatitis are Gray-Turner’s sign and Cullen’s sign. Gray-Turner’s sign is indicative of retroperitoneal hemorrhage. It presents with bruising of the flanks and generally takes at least 24 hours to develop. Cullen’s sign also takes at least 24 hours to appear and is characterized by an ecchymotic region around the umbilicus that has an irregular border. Cullen’s sign is representative of retroperitoneal or intraperitoneal hemorrhage.
Medications that can cause pancreatitis include steroid therapy, furosemide (Lasix), and acetaminophen (Tylenol). A patient presenting with abdominal symptoms, who is on long-term steroid, diuretic, or acetaminophen therapy, should lead to a heightened suspicion of pancreatitis secondary to the medication therapy. Additionally, immuno-compromised pediatric patients may be susceptible to viral and fungal etiologies of pancreatitis.
Cystic fibrosis, although often thought of solelay as a pulmonary affliction, can often compromise the ductal system of the pancreas by causing occlusions and damage due to thick secretions.. In fact, the name itself is derived from the characteristic scarring and cyst formation that presents in the pancreas with this disease. Damage to the pancreas secondary to cystic fibrosis can present a syndrome known as cystic fibrosis-related diabetes (CFRD) which mimics the disease process of common diabetes mellitus.
Diagnosis of pancreatic disease can be made based on a combination of medical history, radiography (ultrasound or radiography), and laboratory studies. The initial blood work performed to monitor pancreatic function will generally include complete blood counts (CBC), a basic chemistry panel, and amylase, lipase, and trypsin levels. Amylase, lipase, and trypsin are pancreas-specific enzymes that show elevations in the presence of pancreatic disease. Additional metabolic system functions will often be included as well (thyroid, liver panel, etc.). Further specific testing for the diagnosis of diabetes mellitus will be discussed later.
Diabetes Mellitus
Diabetes mellitus is the predominant pancreatic disease. Although there are ancillary etiologies (gestational, cystic fibrosis, etc.), diabetes mellitus is classically defined as being either Type 1 or Type 2 in nature. The terms “insulin dependent” and “non-insulin dependent” (IDDM or NIDDM, respectively) are somewhat misleading and are falling out of favor within the medical community becayse many Type 2 diabetics rely on insulin despite having a different disease process than Type 1 diabetics.
Despite the dual etiologies, the end result is the same. The body is unable to convert glucose properly and cells are not able to access this energy. This causes a metabolic crisis, resulting in acidosis, electrolyte imbalance, and, ultimately, death. The specifics of this process are discussed in the following sections.
Type 1 diabetes, often (incorrectly) referred to as juvenile diabetes or juvenile onset diabetes, is an autoimmune disease in which the pancreas does not produce insulin. Treatment for Type 1 diabetes is artificial (endogenous) insulin administration by the patient. Type 1 diabetes is not reversible and requires insulin administration for the rest of the patient’s life. Type 1 diabetes is much less prevalent than Type 2 diabetes and, despite being associated with juvenile onset, it can occur at any age.
Type 2 diabetes, often (incorrectly) referred to as adult onset diabetes, is caused by insufficient amounts of insulin production (as opposed to a lack of insulin production that is characterized by Type 1 diabetes) or by insulin resistance. This form of diabetes is often attributable to unhealthy lifestyle choices by the patient (obesity, high carbohydrate diet, etc.). The severity of Type 2 diabetes varies and can be so mild that changes in lifestyle (weight loss, better diet, etc.,) may curb the manifestation of this disease, or can be so severe that both oral antihyperglycemics and insulin are used for control.
Presentations of new onset (previously undiagnosed) diabetes are based on a hyperglycemic profile. It is important for field providers to realize that hypoglycemia is not an initial presentation of diabetes. Instead, hypoglycemia is a manifestation of the pharmacological interventions that diabetic patients take.
In the pediatric patient, malaise, lethargy, and changes in mood are prominent initial symptoms of diabetes. Weight loss may also occur despite normal or increased appetite (polyphagia). Often, parents might feel that the child has a cold, flu, or other infectious process that “just won’t go away” due to the presence of malaise and abdominal complaints. Hyperglycemia can also inhibit the immune response, therefore, recurrent infection (urinary tract, gynecological, dermatological, etc.) may actually be present.
An approximate blood glucose level of 180 mg/dL is the approximate upper limit for what the kidneys are able to reabsorb for both pediatrics and adults. When a blood glucose level higher than 180 mg/dL occurs, glucose will begin spilling into urine and the hallmark hyperglycemic symptoms of polydipsia and polyuria will begin to present.
Once ketoacidosis develops, symptoms may include abdominal complaints, Kussmaul respiratory pattern, a fruity odor on the breath, symptoms of severe dehydration, and, ultimately, coma. Textbook vital signs for a diabetic ketoacidosis patient are tachypnea, tachycardia, and mild hypotension, although presentation will vary from patient to patient.
Diagnosis of Diabetes
When new onset diabetes is suspected, the traditional triad of diagnostics is a fasting blood glucose test, a random blood glucose test, and a two-hour oral glucose tolerance test. Glucose levels above 126 mg/dL for the fasting test, or 200 mg/dL for the other two tests, is indicative of a probable diabetic condition.
On the forefront of diabetes testing, a newer test for the presence of this condition has been developed known as glycosylated hemoglobin level (HbA1c, or often referred to as an “A1c”). The test evaluates the level of glucose that is bound to the hemoglobin and this level acts as a proxy for the overall blood glucose concentration. The advantage of this test is it provides a long-term view of the situation. The test actually evaluates a picture of what glucose levels have been over the preceding 60 to 90 days and is a more complete view of glycemic levels. An A1c level of 6.5% or higher is positive for a diabetic condition.
Therapy
Along with careful monitoring, the treatment for Type 1 diabetes is insulin administered via multiple daily subcutaneous injections or via an insulin pump. Through pharmacological modification, there are various forms of insulin available to the diabetic. The patient and his or her endocrinologist will create a treatment program that works best for the individual. The various types of insulin available are best classified by their onset and duration of action.
Aspart (NovoLog) and Lispro (Humalog) are rapid-acting forms of insulin with peak actions 30 to 180 minutes after administration. Regular insulin (Humulin R/Novolin R) is a short-acting insulin that takes slightly longer to begin onset than rapid-acting insulin (15 to 60 minutes versus 6 to 30 minutes) and peaks 2 to 4 hours after administration.
NPH (Humulin N/Novolin N) is the most common intermediate class insulin. NPH provides an onset of 2 to 4 hours after administration and peaks anywhere from 4 to 10 hours after administration. Lantus (glargine) is the most common long-acting insulin available. Lantus provides an onset of action 90 minutes to 6 hours after administration and provides steady action for a 24-hour period without a noticeable peak. Lantus is often used in Type 2 diabetic patients requiring insulin.
Combination insulin is also available and is referred to by the ratio of medication involved (i.e., “70/30” has 70% intermediate-acting insulin and 30% rapid-acting insulin). Combination insulins allow patients to obtain the benefits of different types of insulin in concert with another form of insulin to create a comprehensive antihyperglycemic profile.
The term “sliding scale” refers to a patient prescribed to take different amounts of insulin based on their pre-administration glucose level. A patient may take “x” units of 70/30 if his or her sugar is between 100 mg/dL and 150 mg/dL, or “y” units if it is between 150 mg/dL and 300 mg/dL.
Transplantation of the pancreas is another viable option for certain Type 1 diabetics. It is generally reserved for those diabetics termed “brittle,” who have daily difficulty maintaining normal glucose levels despite insulin therapy. This treatment process is still evolving, however, for adults without surgical contraindications, it is proving that patients are able to live without the need for exogenous insulin administration. Strong renal function is required for transplantation due to the metabolic demands of the anti-rejection medications. Unfortunately, renal insufficiency is a common side effect of prolonged diabetes. Accordingly, pancreatic transplantation is often performed in tandem with renal transplantation.
The primary risk associated with insulin therapy is the failure to adequately maintain normal blood sugar levels . On the acute front, insulin-induced hypoglycemia is a critical, fast-occurring, life-threatening condition. Hyperglycemia due to improper diabetes control presents a more chronic yet still life-threatening situation. As with any exogenous medication administration, anaphylaxis is always a possibility. The failure to maintain aseptic technique during administration, medication dosage errors, and improper storage of the medication can all cause insulin-related complications. Alcohol use and concurrent use of other medications (prescription and non-prescription) can affect the effectiveness of insulin.
Type 2 diabetes is a somewhat newer phenomenon among pediatric patients and it is increasing at an alarming pace due to childhood obesity. Treatment for Type 2 diabetes is broader and more complex based on the severity of the disease and may vary from diet control to insulin therapy. Since pediatric Type 2 diabetes is a newer phenomenon, there is still considerable discussion over what is the best algorithm for treatment. For Type 2 diabetics, behavior modification and lifestyle changes will generally be at the forefront of treatment. Behavior modification, with a pediatric patient. presents its own set of challenges and relies heavily on the parents having a firm grasp on the importance of a healthy lifestyle.
For oral antihyperglycemic therapy in the pediatric Type 2 diabetic, metformin (Glucophage) is the most popular therapy with which to begin treatment.11 Metformin acts as an insulin sensitizer and increases peripheral glycemic uptake, while decreasing hepatic glucose output. Importantly, it is not often associated with hypoglycemic episodes.
Often, metformin on its own will not adequately control glycemic levels over the long term. If a long-acting insulin is needed, Lantus is usually appropriate for this scenario. Type 2 diabetes is a progressive disease, therefore, what works as treatment of the patient today might not work two years from now. Ongoing monitoring of pharmacological efficacy is required to maintain adequate disease control.
The risks associated with Type 2 diabetes are similar to Type 1 diabetic treatments. Achieving the balance between medications that will prevent hypoglycemic incidents and that will adequatlely lower blood glucose levels is paramount. Since the etiology of Type 2 diabetes is largely attributable to overall health (versus the autoimmune syndrome causative in Type 1 diabetics), achieving normal weight and managing a healthy diet are critical for long-term success in Type 2 diabetic treatment.12
Long-term, chronic risks of the disease include a myriad of life-threatening complications including glaucoma, renal failure, cardiovascular disease, peripheral vascular disease, wound care difficulties, and neuropathies. Proper education of these complications for the diabetic is important in preventing their onset and alerting the patient to the importance of maintaining a normal blood glucose level (euglycemia).
Pre-hospital Emergent Diabetic Presentations
The presentation of diabetic patients for pre-hospital providers may be obvious, such as a hypoglycemic episode in a known diabetic taking insulin, or it may be a more subtle presentation, such as a patient who is having trouble maintaining appropriate glycemic control with oral antihyperglycemics presenting with abdominal complaints.
While there is no specialized physical assessment for known (or suspected) diabetics, you should pay particular attention to mental status, respiratory pattern, cardiovascular status, and overall appearance. Although there is a very large population of diabetics in the U.S., there is a much larger population that does not even know that they are diabetic. If the patient does not regularly see a healthcare professional, your assessment may be the first time a possible diabetic condition is noticed.
The real key to assessing a diabetic, or possible diabetic, is the focused history in which a proper investigation can reveal a myriad of clues that can help bring the situation into focus. Look for culprits that may indicate a change in the patient’s diabetic balance such as increased stress, non-compliance, a change in medications, a recent infection, or weight changes.
If the patient is not already diagnosed as a diabetic, questions focused on recent intake/output, family medical history, energy levels, abdominal symptoms, and changes in weight will be important to ask. A chief complaint of an infectious process may be the only complaint the patient exhibits, therefore, it will be imperative to take an attentive and detailed history to uncover a metabolic cause.
While different pre-hospital systemergency medical personnel have different diagnostic tools at their disposal, this is meant to be a comprehensive list. Pediatric patients are notorious for being able to hemodynamically compensate for a marked amount of time and then decompensate rapidly. It is important to pay special attention to subtle changes in the patient’s cardiovascular status that may indicate impending collapse. Serial vital signs, blood glucometry, EKG, capnography, and point of care (POC) blood chemistries can all be helpful in assisting with differentiating presentations. With the exception of glucometry, many or all of the remaining diagnostics may present as unremarkable despite an underlying diabetic condition.
Hyperglycemia
Hyperglycemic patients can be classified as new onset/not-yet-diagnosed diabetics, or as patients that have been previously diagnosed with diabetes. Clearly, a patient with a known diabetic history is going to cause a provider to focus on a diabetic-related problem more promptly than a new onset diabetic patient.. Causes of hyperglycemia include new onset diabetes, non-diabetic pancreatic disease (discussed in previous sections), changes in medication, infection, stress, weight gain/loss, and non-compliance.
Many pediatric diabetic patients may present with normal vital signs, depending on the severity of the presentation. Depending on the progression of the hyperglycemia and the degree of dehydration that has set in, tachycardia, tachypnea, and/or hypotension may be present.
Diabetic ketoacidosis (DKA) is a life-threatening medical emergency that can occur in the hyperglycemic patient and is present in twenty-five percent of new onset diabetic cases. Forty percent of DKA cases are due to an underlying infection (Klebsiella pneumonia is the most common underlying infection), making it the leading cause of DKA. The next most common cause of DKA is missed insulin doses which may be due to intentional non-compliance, poor storage of medication, or mechanical problemergency medical personnel with the insulin pump.
When the body does not have insulin to assist in using glucose, the body goes into starvation mode and begins to burn fat for fuel. An acidic byproduct of this fat breakdown is ketones. As ketones accumulate in the body, beyond its intrinsic ability to eliminate them, they begin to spill into urine (ketonuria) and create metabolic acidosis. Respiratory compensation (Kussmaul respiration) responds to the acidosis. Already subject to a shift in potassium, DKA can induce vomiting which further aggravates the balance of electrolytes in the body.
The triad of metabolic acidosis, osmotic diuresis, and serum hyperosmolarity cause a critical metabolic cascade within the body. Despite the possibility of hyperkalemia initially, ultimately, the extracellular hydrogen ions associated with acidosis will exchange with intracellular potassium. As the potassium shifts extracellularly, it falls victim to the rampant diuresis associated with this condition. By the time the patient presents with a laboratory value of serum hypokalemia, the patient is in a critical state. The patient with critical serum hypokalemia is at risk for fatal cardiac dysrhythmias, therefore, potassium replenishment must be initiated promptly. Sodium is also affected by the process of DKA. Water is pulled into the extracellular space due to the osmolar and diuretic action, thus, causing dilutional hyponatremia.
From a cardiac standpoint, tachycardia is the typical presenting rhythm in pediatric DKA patients. Patients are prone to prolonged QT intervals and the dysrhythmias that can result from a long QT interval. Lengthening of the QT interval may actually occur with or without hypo/hyperkalemia. All DKA, or suspected DKA patients, require cardiac monitoring. Although definitions vary slightly, typical laboratory values indicative of a DKA diagnosis include a serum blood glucose of >300 mg/dL, a bicarbonate level less than 15 mEq/L, and a pH less than 7.30 which is accompanied by ketonemia and ketonuria.
Cerebral edema is a mysterious phenomenon that occurs in pediatric DKA patients. It is the most common cause of death among pediatric DKA patients and occurs approximately one percent of the time. The etiology of this condition is still not understood. In the past, it was thought to be caused by overaggressive fluid administration, osmolar changes, and serum hyponatremia. However, this theory is frequently called into question by the medical community. Aspects regarding cerebral edema that are never questioned is that itdevelops quickly and ultimately leads to herniation and death. CT scanning is the primary means of diagnosis, however, even that is not entirely reliable as there have been cases where edema had developed without radiological proof.
It is crucial for care providers to notice the initial neurologic symptoms that accompany cerebral edema. Changes in mentation, incontinence, and increased emesis, particularly after treatment has been initiated, should cause heightened concern of developing neurologic catastrophe. The Glasgow Coma Score does not provide a platform sensitive enough to use as a sole means of monitoring mentation changes in these patients. The changes are very subtle and may be as passive as increasing irritability in a patient otherwise thought to be improving.
Additionally, changes in vital signs in line with increased intracranial pressure, most notably relative bradycardia, should be noticed. Often, care providers do not key in to these changes because the patient was already tachycardic, therefore, a decrease in heart rate may now result in a “normal” pulse rate. However, this needs to be interpreted as bradycardic for the patient’s condition. The same is true with tachypnea, since DKA patients frequently present with tachypnea. Although normally a sign of increasing intracranial pressure, increased hypertension is not common with the development of cerebral edema development among the pediatric population.
One of the most common presentations for DKA-induced cerebral edema is for the patient to appear to be improving overall in accordance with the prescribed treatment plan and then to deteriorate unexpectedly and rapidly due to cerebral edema. Despite the fact that most cases of cerebral edema in DKA patients occur after treatment has started, there have been occasional pretreatment episodes of cerebral edema.
If cerebral edema is suspected or identified, fluid therapy should be stopped and the head of the bed should be elevated to at least a 30-degree angle. Intravenous administration of the osmotic diuretic, mannitol, is the primary treatment modality for cerebral edema. Administration of hypertonic saline solution is also an appropriate therapy. These therapies are generally initiated in the hospital but may be present for EMERGENCY MEDICAL PERSONNEL providers in the case of inter-facility transports of such patients. Due to the questionable causes of cerebral edema in pediatric DKA patients, it is not surprising that there has not been overwhelming evidence that one treatment (hypertonic saline or mannitol) is preferable to the other. If cerebral edema worsens, intubation, with or without rapid sequence induction (RSI), may become necessary due to increasing intracranial pressure and a decreasing level of consciousness.
Caution must be exercised when using succinylcholine with hyperkalemia and an iatrogenic worsening. The patient is breathing quickly for a reason. If you take over their airway and bag them at a normal rate, the pH may worsen. Hyperventilation may be appropriate in some cases. Consult medical control and your local protocols.
Treatment for DKA is not done rapidly, but is done steadily over the course of a few days. The management of DKA so delicate that the adage is it is best to gear DKA management to “keep the patient a little sweet and a little dry.” Correction of immediate life threats is addressed and then a gradual correction of metabolic deficiencies and volume is undertaken.
Initial volume replacement follows the traditional hypovolemia algorithm for pediatrics with the use of a bolus of 0.9% normal saline solution at a rate of 10 mL/kg over the first hour. In the presence of normal serum sodium levels, further fluid administration can be accomplished with the use of hypotonic saline solution over the next 48 hours. Although dependent on the degree of hypovolemia , a goal of 70mL/kg of total replacement is a common benchmark set for a 48-hour period.
Once fluid replacement is initiated and renal function is verified, hypokalemia needs to be corrected immediately via infusion. Insulin administration causes potassium levels to drop and,consequently, the administration of insulin in conjunction with existing hypokalemia can be fatal. Insulin (generally a therapy initiated in hospital) may or may not be given as an initial bolus but will always be included as an ongoing infusion at a slow and gradual pace once hypokalemia is corrected. Infusion rates for insulin are usually set at 0.05 to 0.10 kg/hr for short-acting insulin. The presence of insulin infusions may be present for EMERGENCY MEDICAL PERSONNEL providers in various inter-facility transport scenarios.
Once blood glucose levels are below 300 mg/dL, glucose solution is added to the treatment plan to maintain a conservative level of blood glucose decline. Despite the presence of metabolic acidosis, sodium bicarbonate administration is generally not part of the standard pre-hospital DKA treatment plan. A ph less than 7.0 may be indicative of the need for sodium bicarbonate in the definitive care setting. Aggressive monitoring of laboratory values and changes in patient status are mandatory due to the complex treatment regimen initiated on these patients.
Prior to arrival at the hospital (in the absence of laboratory values), management of hypovolemia, cardiac dysrhythmias, and astute monitoring of mental status changes are mainstays of treatment. However, pre-hospital providers performing an inter-facility transport, or those working in advanced practice systemergency medical personnel with POC laboratory testing, may be involved with more complex treatment. Astute identification of the onset of cerebral edema by a pre-hospital provider may be the most important factor throughout the entire course of treatment for the patient.
Hyperosmolar Nonketotic State
Hyperosmolar nonketotic state (HNS) is an unusual complication that can occur in Type 2 diabetes and, on very rare occasions, can occur in Type 1 diabetics as well. HNS is important because of the rapid increase in Type 2 diabetes in the pediatric population. This condition has also been referred to as hyperosmolar nonketotic coma (HONC). Historically, HNS was associated with older, frail, Type 2 diabetics. However, with the changing face of diabetes shifting toward youth, this condition may be a possibility in younger patients.
HNS is differentiated because it is a nonketotic syndrome. There is not a complete absence of insulin in these patients (Type 1) that would be a catalyst for rampant ketone buildup. Diagnostic criteria for HNS are a blood glucose level of 600 mg/dL, a pH of 7.3, a bicarbonate level greater than 15 mEq/L, signs of severe dehydration, and the absence of ketones. Note the lack of acidosis or ketosis.
The symptoms of HNS are nearly identical to DKA but with a longer onset period. The history may be subtly different which may include a history of frequently drinking sugary drinks to cope with increased thirst. The treatment regimen for these patients is parallel to standard DKA treatment, however, the etiology of the syndrome is different.
Hypoglycemia
There are many obscure causes of pediatric hypoglycemia, however, this article focuses on those related to diabetic patients. Complications from diabetes are the most common cause of pediatric hypoglycemia.
Hypoglycemia, in the pediatric diabetic patient, is generally caused by a mismatch in eating, exercise, and medication administration. This already complicated routine is even more complicated for a pediatric patient due to the difficulty in managing the adult responsibilities of the disease. For these reasons, pediatric diabetics are more susceptible to hypoglycemic episodes than adult diabetics.
The body is highly sensitive to decreasing blood glucose levels. Although variation exists, once blood sugar starts to drop below 80 mg/dL, a physiological response begins. Even mild hypoglycemia can cause symptoms such as irritability, anxiety, weakness, hunger, and trembling. The signs and symptoms of hypoglycemia in the pediatric can be subtle and highly focused on behavior. Staring spells, poor concentration, nightmares, inappropriate crying, short-term memory dysfunction, and visual/hearing disturbances are all symptoms of pediatric hypoglycemia.
In a non-diabetic patient, the initial response to lowering blood sugar levels is the body’s attempts to decrease insulin secretion by the pancreas. As insulin levels are lowered, the kidneys and liver are stimulated to increase glucose production. The next response is multifaceted. Epinephrine and norepinephrine are released to increase renal and hepatic glucose production and limit peripheral glucose utilization via peripheral vasoconstriction. The release of these catecholamines is one of the prime reasons that a patient will feel anxious, nervous, and will be tachycardic. Simultaneously, glucagon is released to stimulate hepatic glucose production via glycogenolysis. In younger patients or those with hepatic compromise, this process can be limited by the availability of glycogen stores..
The previously discussed metabolic responses to hypoglycemia are designed for the short-term and immediate resolution of hypoglycemia. The body also releases cortisol, a growth hormone, to counter hypoglycemia over a longer period. Cortisol is produced by the adrenal gland and, when secreted, aids in increasing blood sugar by increasing glycogen synthesis (glycogenesis). It also assists in protein, fat, and carbohydrate metabolism. Growth hormones are released by the pituitary gland by command of the hypothalamus, thus, reducing glucose uptake in muscles and stimulating glucose production.
If blood glucose levels progress still lower, more severe neurological symptoms develop including significant altered mental status, seizure activity, combative behavior, and coma. If prolonged and severe hypoglycemia persists, permanent neurological insult is possible.
The body’s response to hypoglycemia can be highly effective in the non-diabetic patient. However, with the diabetic patient administering insulin or oral antihyperglycemics, the entire process is subverted via exogenous medication administration. Additionally, glucagon production in the Type 1 diabetic patient is blunted. This means that the Type 1 diabetic is naturally poorly equipped to defend against hypoglycemia.
Generally, in conjunction with an appropriate clinical presentation, significant hypoglycemia is considered a blood glucose level below 60 mg/dL for pediatrics or 45 mg/dL for infants. These blood glucose levels are not definitive and noticeable symptoms may be noticed above these levels.
Pre-hospital Treatment
The mildly hypoglycemic patient presents with the challenge of halting the progression of hypoglycemia and correcting the blood sugar levels in a mild manner. The moderate and severe hypoglycemic patient presents multiple challenges to the pre-hospital provider. Among these challenges include protecting the patient from self-injury, managing the patient’s airway, and facilitation of parenteral medication administration to reverse hypoglycemia in addition to attempting to restore normal glucose delivery to the body via parenteral medication administration.
If a patient has an appropriate mental status and can manage his or her airway, oral glucose administration of approximately 15 g of oral glucose paste is the appropriate treatment. Intake of high-sugar juices or soda may also be used for this purpose. Pure glucose is the ideal treatment and eating proteins or high fat foods will delay the efficacy of absorption. After 15 to 20 minutes of consumption, the patient should recheck his or her blood sugar levels to confirm a return to normal. The possibility of rebound hypoglycemia needs to be addressed with the patient and caretaker.
For a severe hypoglycemic patient presenting with an altered mental status, administration of intravenous dextrose is the treatment of choice. The dosages and concentrations of pediatric dextrose administration vary according to different EMERGENCY MEDICAL PERSONNEL agencies and medical controls. Dextrose is always given at lower concentrations to the pediatric patient than to the adult patient. Many EMERGENCY MEDICAL PERSONNEL systemergency medical personnel will use 25% dextrose concentrations for all pediatric hypoglycemic patients, while others will incorporate 10% dextrose concentrations for infant patients. Dextrose dosage recommendations vary between 200 and 500 mg/kg and are usually gauged via pediatric medical dosage tape (i.e., Broslow).
If intravenous access can not be obtained in the field, intramuscular glucagon, at a dosage of 0.1 mg/kg not to exceed 1 mg, is a secondary choice for treatment. Post-administration nausea is possible. Patients deemed susceptible to hypoglycemia will frequently have an at-home glucagon kit for administration by family members. Glucagon itself is not a sugar, it is a pancreatic hormone that can raise blood sugar levels. Insufficient glycogen stores, as may be present in pediatrics and alcoholics, will limit the efficacy of glucagon administration.
With any correction of hypoglycemia, the goal is to return blood glucose levels to normal without shocking the system into hyperglycemia. After correcting hypoglycemia, it is important to prevent against rebound hypoglycemia by having the patient eat a meal with complex carbohydrates, such as a sandwich with peanut butter.
The decision on whether a patient requires transport is somewhat more complex when faced with a pediatric patient. The decision needs to be made with the patient’s family and a proper post-hypoglycemic incident plan needs to be created. If there is any doubt that the family and patient will be able to manage the situation appropriately upon EMERGENCY MEDICAL PERSONNEL exiting, then the patient is better suited to be transported to an emergency department.
While the critical phase of the hypoglycemic event may have passed, the need for follow-up physician involvement is advisable due to the complexities of pediatric diabetes. Follow up care may be with the patient’s endocrinologist or with an emergency facility.
One of the primary considerations after a hypoglycemic episode is eliciting a cause for the event. If they took insulin and didn’t eat, this is not a major medical concern. If they are ill and cannot eat or took too much oral hypoglycemic or long acting insulin, they need to be transported. You should pay particular attention to the patient if frequent hypoglycemic events are occurring and/or the patient is having difficulties in maintaining proper blood glucose levels on a regular basis.
References
Internet: Center for Disease Control. National Diabetes Fact Sheet. Center for Disease Control Diabetes Website. Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed November 14, 2011.
Internet: Boyles, S. Type 1 Diabetes May Double in Young Kids. WebMD Health News Website. Available at http://diabetes.webmd.com/news/20090527/type-1-diabetes-may-double-in-young-kids. Accessed November 16, 2011.
Journal Article: Portuese, E., Orchard, T. Mortality in Insulin-Dependent Diabetes. In: Diabetes in America, 2nd Edition. Bethesda, MD: NIDDK. 1995:221-232.
Journal Article: Vaughn, D. D., Jabra, A. A., Fishman, E. K. Pancreatitis in Children and Young Adults. Scientific Exhibit. 1998. 18:1171-1187.
Journal Article: Locasto, D. A., Calhoun, D. J., Meek, R. J., et al. Distinguishing Diabetes: Differentiate between Type 1 & Type 2 DM. JEMERGENCY MEDICAL PERSONNEL. November 2009.
Internet: Kishore, P. Diabetes Mellitus: Merck Manual Website. Available at http://www.merckmanuals.com/home/hormonal_and_metabolic_disorders/diabetes_mellitus_dm/diabetes_mellitus.html. Accessed January 9, 2012.
Internet: Raghavan, V. Diabetic Ketoacidosis Clinical Presentation. Medscape Website. Available at http://emedicine.medscape.com/article/118361-clinical#aw2aab6b3b2. Accessed January 12, 2012.
Internet: Jospe, N. Diabetes Mellitus in Children. Merck Manual Website. Available at http://www.merckmanuals.com/home/childrens_health_issues/diabetes_mellitus_inchildrendm/diabetes_mellitus_in_childrendm.html. Accessed November 19, 2011.
Journal Article: American Diabetes Association. Insulin Administration. Diabetes Care. 2003. 26:S121-124.
Internet: UCLA. Pancreas Transplants. UCLA Health System, Transplantation Services Website. Available at http://transplants.ucla.edu/body.cfm?id=81. Accessed November 15, 2011.
Journal Article: Gahagan, S., Silverstein, J. Prevention and Treatment of Type 2 Diabetes in Children. Pediatrics. 2003. 112:e341.
Journal Article: Copeland, K. C., Becker, D., Gottschalk, M., et al. Type 2 Diabetes in Children and Adolescents. Clinical Diabetes. 2005. 23:181-185.
Journal Article: Munk, M. D. Pediatric DKA. JEMERGENCY MEDICAL PERSONNEL. October 2008.
Journal Article: Rosenbloom, A. L., Schatz, D. A., Krischer, J. P., et al. Prevention and Treatment of Diabetes in Children. J Clin Endocrinol Metab. 2000. 85:509-513.
Journal Article: Umpierrez, G. E., Murphy, M. B., Kitabchi, A. E. Hyperglycemic Crises in Diabetics. Diabetes Care. 2004. 27:S94-S102.
Journal Article: Kitabchi, A. E., Wall, B. M. Management of Diabetic Ketoacidosis. Am Fam Physician. 1999. 60:455-464.
Journal Article: Glaser, N. S., Wootton-Gorges, S. L., Mrcin, J. P., et al. Mechanism of Cerebral Edema in Children with Diabetic Ketoacidosis. J Pediatr. 2004. 145:164-171.
Internet: Krane, E. J. Diabetic Ketoacidosis and Cerebral Edema. Stanford University Pediatrics and Anesthesiology Website. Available at http://pedsccm.org/File-Cabinet/Metab/DKA-CEdema.html. Accessed November 13, 2011.
Journal Article: Roberts, M. D., Slover, R. H., Chase, H. P. Diabetic Ketoacidosis with Intracerebral Complications. Pediatric Diabetes. 2001. 2:109-114.
Journal Article: Fowler, M. J. Hypoglycemia. Clinical Diabetes. 2008. 26:170-173.
Journal Article: Umpierrez, G. E., Murphy, M. B., Kitabchi, A. E. Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Syndrome. Diabetes Spectrum. 2002. 15:28-36.
Journal Article: Clarke, W., Jones, T., Rewers, A., et al. Assessment and Management of Hypoglycemia in Children and Adolescents with Diabetes. Pediatric Diabetes. 2009. 10:134-135.
Journal Article: Socransky, S. J., Pirrallo, R. G., Rubin, J. M. Out-of-hospital Treatment of Hypoglycemia: Refusal of Transport and Patient Outcome. Acad Emerg Med. 1998. 5:1080–1085.
Internet:Childhood Diabetes. Joslin Diabetes Center Website. Available at http://www.joslin.org/info/childhood-diabetes.html. Accessed August 7, 2014/
Internet: Children and Diabetes. CDC Website. Available at http://www.cdc.gov/diabetes/projects/cda2.htm. Accessed August 7, 2014.


